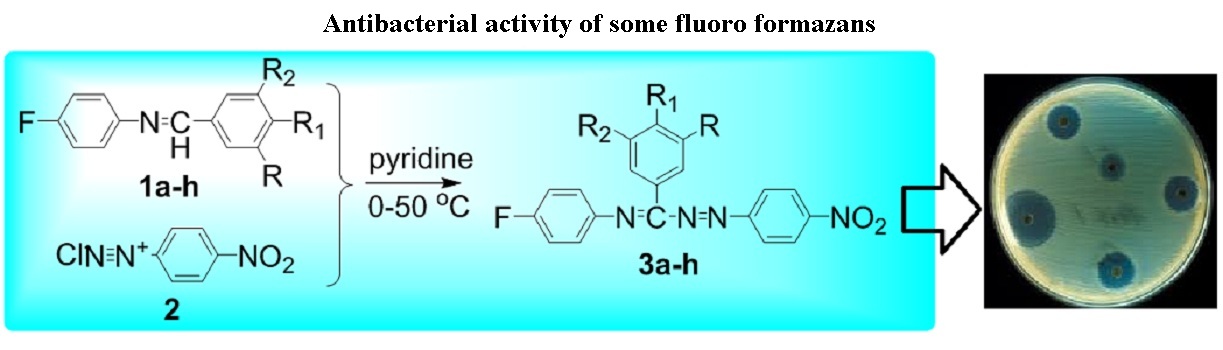
Synthesis and Antimicrobial Evaluation of Some New Fluoro Formazans
Abstract
References
[1] Shawali A.S., Samy N.A., J. Adv. Research 2015, 6,241-254
[2] Bharadwaj S.D., Asian J.Chem.1998, 10, 39-42
[3] Mariappan G., Korim R., Joshi, N.M., Alam F., Hazarika R., Kumar D., J. Adv. Pharm Tech. Res. 2010, 1(4), 396-400.
[4] Nadendla R,R., Mukkani, K., Rao,G,S., Babu A.N. Curr. Trends Biotech pharm, 2010, 4, 545-550.
[5] Raval J.P., Patel P. , Patel P.S., Int .J Ppharm. Tech. Res. 2009, 4, 1548-1553.
[6] Misra V.S., Dhar S., Chowdhary B.L. Pharmazie. 1978, 33, 790-802
[7] Stefanescu E., Dorneanu M., Danila C., Aprotosoaie C, Grosu G., Pavelescu M. Rev.Med. Chir Soc Med Nat IASI, 1997, 101, 178-82
[8] Bhardwaj S.D., Jolly V.S., Asian J.Chem. 1997, 9, 48-51.
[9] Marjadi S.I., Solanki J.H., Patel A.L. J. Chem. 2009, 6, 844-848.
[10] Chavan S.B. , Zangade S.B., Vibhute A.Y., Vibhute Y.B. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 262-269.
[11] Kumala prasad S.A., Subrahmanyam E.V.S., Shabaraya A.R. World. J. Pharm. Res. 2014, 3, 2741-2752.
[12] Khanna R., Palit G., Srivastava V.K., Shankar K. Indian J. Chem. 1990, 293, 556-560.
[13] Kumar A., Verma R.S., Bhatia S.K., Gupta S., Jetty V.K., Bajpai S.K. Pak. J. Sci. Ind. Res. 1996, 39, 1-4.
[14] Yoghoglu A.S., Senoz H. Hacettepe J. Biol. Chem. 2013, 41, 371-380.
[15] Hagmann W.K. J. Med. Chem. 2008, 51, 4359-4369
[16] Ismail F.M.D. J. Fluorine Chem. 2002, 118,27-33.
[17] Karthikeyan M.S., Holla B.S., Kumari N.S., Eur. J. Med. Chem. 2007 ,42, 30-36.
[18] Park B.K., Kitteringham P.M., Veil. O. Annu. Rev. Pharm. Toxicol. 2001, 41, 443-470.
[19] Nelson J.M., Chiller T.M., Puwers J.M, Angulo F.J. Clin. Infect Dis., 2007, 44, 977-980.
[20] Shinde A.T., Zangade S.B., Chavan S.B. Vibhute Y.B American J. Pharm. Tech. Res. 2011, 1, 43-48.
[21] Benson H.J., Microbiological apllications W.C. Brown publications, Boston, M.A, 1990.
Refbacks
- There are currently no refbacks.