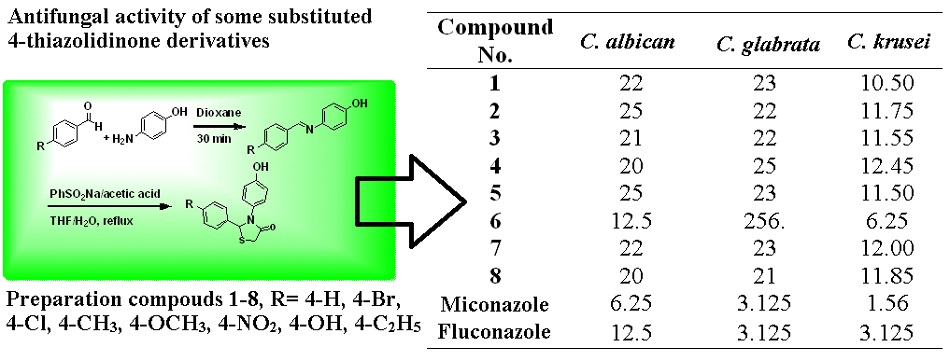
Synthesis, Characterization and Antifungal Activity of Some Substituted 4-thiazolidinone Derivatives
Abstract
References
P. Shiva, S. Surenda, R. Krishna, I. Virgil. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981, 81 (2), 175–203.
[2] S. Kucuzel, E. Oric, S. Rollas, F. Shahin, A. Ozbik . Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Euro. J. of Med. Chem. 2002 ,37(3), 197-206.
[3] V. Gududuru , E. Hirh , J.Dalton ,D.Miller. Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorganic & Med. Chem Letters. 2004,14(21), 5289-5293.
[4] A. Ameya , R. Chavan , R. Pai. Synthesis and Biological Activity of N-Substituted-3-chloro-2-azetidinones. Molecules. 2007, 12(11), 2467-2477.
[5] S. Bondok ,W. Khalifa , A. Fadda. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinones and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Euro. J. of Med. Chem. 2007, 42(7) , 948-954.
[6] F, Aydogan, N. Ocal, S. Turgut, C.Youlcan. Transformations of Aldimines Derived from Pyrrole-2-carbaldehyde. Synthesis of Thiazolidine -Fused Compounds. Bull. Korean Chem. Soc. 2001, 22(5) , 476-480.
[7] A. Kumar Jain, A.Vadiya, V. Ravchindran, S. Kumar Kashaw, R. Aggarwal. Recent developments and biological activities of thiazolidinones derivatives. Bioorganic & Med. Chem. 2012 , 20(11), 3378-3395.
[8] M. Abhinit, M. Ghodke, N. Partima. Exploring Potential of 4-Thiazolidinones. International Journal of Pharmacy and Pharmaceutical Sciences. 2009, 1(1), 47-64.
[9] M. Cacic, M. Molnar, B. Sarkanj, E. Has-schon,V.Rajokovic. Synthesis and Antioxidant Activity of Some New Coumarinyl-1,3-Thiazolidine-4-ones. Molecules, 2010, 15(10), 6795-6809.
[10] D. Havrlicuc, B. Zimenkovesky, O.Vaselenko, C.W.Day, F.Smee, P.Grellier, R. Lesyk. Synthesis and biological activity evaluation of 5- pyrrazoline substituted 4-thiazolidinones. Euro. J. of Med. Chem. 2013, 66, 228-237.
[11] D. Bhot, R. Khont, V. Shankhavra, H. Parekh. Synthesis of Some New Heterocyclic Compounds with Potential Biological Activity. Journal of Sciences, 2006 , 17(4), 323-325.
[12] S. Wadher, N. Karande, S. Sonawane, P.Yeloe. Synthesis and Biological Evaluation of Schiff Base and 4- thiazolidinones of Amino salicylic acid and Their Derivatives as Antimicrobial Agent. International of Chem Tech Research .2009, 1(4), 1303-1307.
[13] S. Chandrappa , S. Prasad, K.Vinaya, C. Kumar, K. Ranjappa. Synthesis and in vitro antiproliferative activity of 5-(4-methyl-benzylidene)-thiazolidine-2,4-diones. Investigational New Drags. 2008, 26(5), 437- 444.
[14] S. Ozkirimli, F. Kazan, Y.Tunali. Synthesis, antibacterial and antifungal activities of 3-(1,2,4-triazol-3-yl)-4-thiazolidinones. Journal of Enzyme Inhibition and Medicinal Chemistry. 2009, 24(2), 447- 452.
[15] S. Kasmi-Mir, A. Djafri, L. Paciun, J. Hamelin, M. Rahmouni. One-Pot Synthesis of 5-Arylidene-2-Imino-4-Thiazolidinones under Microwave Irradiation. Molecules, 2006, 11(8), 597-602.
[16] N. Siddiqui, M. Arshad, W. Ahsan, M. Alam. A Valuable Insight into the Recent Advances and Biological Activities. Inter. J. of Pharm. Sci. and Drug Research.2009,1(3),136-143.
[17] W, Al-Sayed, Y. Abed-Momen, H. Abed-Alrahman. Antimicrobial Activity of New 2,4-Disubstituted Thiazolidinone Derivatives. Z. Naturforsch. 2009,64,785-789.
[18] W. Shoubing, Y, Zhao, G. Zhang, Y. Lv, N. Zhang, P. Gong. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Euro. J. of Med. Chem. 2011,46(8), 3509-3518.
[19] T. Srivstava, K. Gaikwad, W. Haq, S. Sinha, S. Katti. Synthesis and biological evaluation of 4-thiazolidinone derivatives as potential antibacterial agents. Arkivoc,2005,2, 120-130.
[20] R. Haulser, J. Haltom, S. Powels. Correlation between membrane effects and resistance to graminicides. Plant Physiology ,1997, 1,1035-1043.
Refbacks
- There are currently no refbacks.