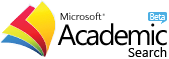Hydrogenation/Deoxygenation (H/D) Reaction of Furfural-Acetone Condensation Product using Ni/Al2O3-ZrO2 Catalyst
Abstract
References
[1] A. Corma, O. de la Torre, M. Renz, N. Villandier, Angew. Chem. Int. Ed. 2011, 50, 2375–2378.
[2] A. Corma, S. Iborra, A. Velty, Chem. Rev. 2007, 107, 2411–2502.
[3] G. W. Huber, J. N. Chheda, C. J. Barrett, J. A. Dumasic, Science 2005, 308, 1446–1449.
[4] X. Zhang, Q. Zhang, T. Wang, L. Ma, Y. Yu, L. Chen, Bioresour. Technol. 2013, 134, 73–80.
[5] L. Faba, E. Díaz, S. Ordóñez, Appl. Catal. B Environ. 2012, 113-114, 201–211.
[6] O. Kikhtyanin, V. Kelbichová, D. Vitvarová, M. Kubů, D. Kubička, Catal. Today 2014, 227, 154–162.
[7] M. Hellinger, H. W. P. Carvalho, S. Baier, D. Wang, W. Kleist, J.-D. Grunwaldt, Appl. Catal. Gen. 2015, 490, 181–192.
[8] H. Shafaghat, P. Sirous Rezaei, W. M. A. W. Daud, RSC Adv 2015, 5, 33990–33998.
[9] L. Faba, E. Díaz, S. Ordóñez, Appl. Catal. B Environ. 2014, 160-161, 436–444.
[10] D. C. Elliott, Energy Fuels 2007, 21, 1792–1815.
[11] V. N. Bui, D. Laurenti, P. Delichère, C. Geantet, Appl. Catal. B Environ. 2011, 101, 246–255.
[12] N. Rahemi, M. Haghighi, A. A. Babaluo, M. F. Jafari, P. Estifaee, J. Ind. Eng. Chem. 2013, 19, 1566–1576.
[13] S. J. Han, Y. Bang, J. Yoo, K. H. Kang, J. H. Song, J. G. Seo, I. K. Song, Int. J. Hydrog. Energy 2013, 38, 15119–15127.
[14] S. J. Han, Y. Bang, J. Yoo, J. G. Seo, I. K. Song, Int. J. Hydrog. Energy 2013, 38, 8285–8292.
[15] S. M. Ulfa, I. Sari, C. P. Kusumaningsih, M. F. Rahman, Procedia Chem. 2015, 16, 616–622.
[16] J. Luo, L. Arroyo-Ramírez, J. Wei, H. Yun, C. B. Murray, R. J. Gorte, Appl. Catal. Gen. 2015, 508, 86–93.
[17] Y. Li, X. Huang, Q. Zhang, L. Chen, X. Zhang, T. Wang, L. Ma, Appl. Energy 2015, 160, 990–998.
[18] Z. He, X. Wang, Catal. Sustain. Energy 2012, 1, DOI 10.2478/cse-2012-0004.
Refbacks
- There are currently no refbacks.